Research
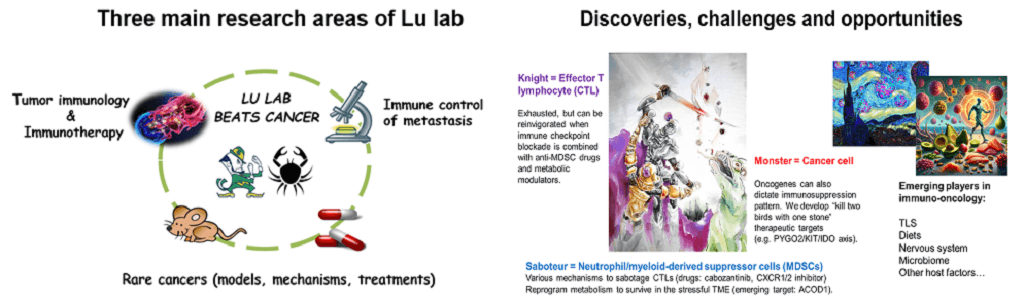
The research goal of the Lu lab is to develop novel insights into the genomic, genetic and molecular mechanisms of tumor immunology, organotropic metastasis and treatment resistance, and apply such knowledge to develop and improve therapeutic interventions to benefit cancer patients. Our laboratory at University of Notre Dame is focused on identifying cancer cell intrinsic and extrinsic mechanisms of tumor immune evasion, in various advanced malignancies, including prostate, breast, kidney, pancreatic, and penile.
- Our recent publications firmly establish that immunosuppressive myeloid cells, especially tumor-associated neutrophils (also known as polymorphonuclear myeloid-derived suppressor cells, PMN-MDSC), play the predominant role in inducing the exhaustion of cytotoxic T lymphocytes (CTL) and distant metastasis of prostate cancer and penile cancer.
- Our research results also reveal a number of targetable mechanisms on how the oncogenic signaling in neoplastic cells exerts the cell non-autonomous functions to control the cancer-immune interactome in solid tumors.
- Our laboratory is interested in developing chemical or biologic agents and dietary interventions as emerging cancer therapies. Examples include small-molecule inhibitors of the chromatin effectors, antibody-drug conjugates (ADC), CAR-NK cells, and ketogenic supplement.
- Our research approach integrates genetically engineered mouse models, functional genomics, experimental therapeutics, and cutting-edge experimental and computational methodologies (single cell RNA-seq, CyTOF, high-throughput drug and CRISPR/cas9 screen, molecular digital pathology, multi-omics integration, etc.).
Tumor Microenvironment and Combination Immunotherapy
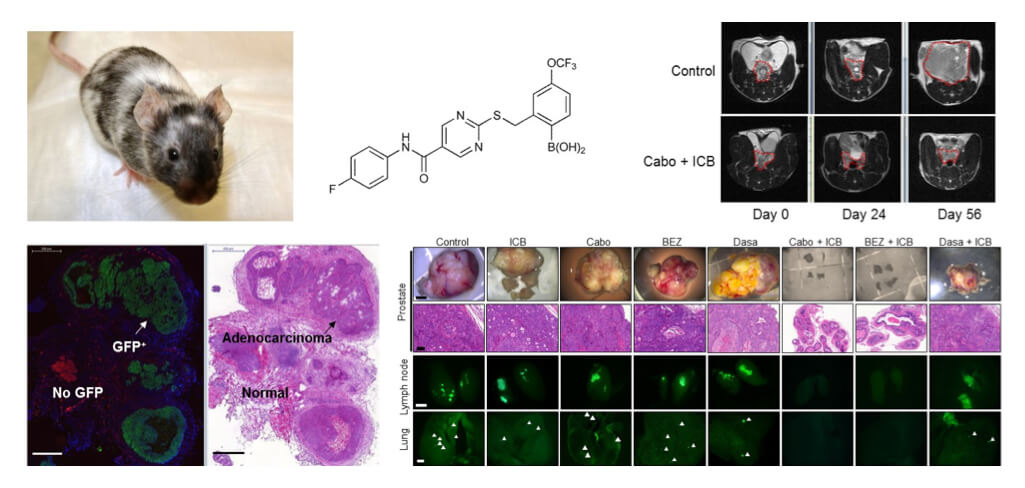
Understanding and targeting the tumor microenvironment is at the forefront of current basic and translational cancer research. Targeting tumor microenvironment is closely related to tumor immunology and immunotherapy, one of the most exciting and rapidly evolving areas of cancer research. An intense focus of research in our lab is to investigate the molecular and cellular mechanisms underlying the cancer ─ tumor microenvironment crosstalk, in particular interactions between cancer cells and the myeloid compartment, in both primary tumors and metastases to bone and other organs. We hypothesize that the efficacy of immune checkpoint blockade drugs (e.g. anti-CTLA4, anti-PD1 antibodies) on refractory metastatic cancer can be potently enhanced when combined with other therapy modalities, including molecularly targeted therapy that specifically antagonize myeloid-derived suppressor cells (MDSCs) yet preserve T cell functions in the tumor microenvironment. We have shown preclinical evidence attesting to this hypothesis (Cancer Discovery, 2016; Nature, 2017). We are now taking omics and target-based approaches to in-depth understanding of the myeloid compartment of the tumor signaling network, and using the knowledge we gained to guide new therapeutic design for more effective and less toxic combination immunotherapy. We mainly focus on prostate cancer and breast cancer, but the mechanisms we unveil can apply to other cancer types.

Metaphor for Combination Therapy
In an ancient idiom from Tao, while the mantis stalks the cicada, the mantis is being attacked by the oriole from behind. In an immunogenic tumor microenvironment, the tumor cell, cytotoxic T cell and immunosuppressive myeloid cell are metaphorical to the cicada, mantis and oriole, respectively. Therapies targeting immumosuppressive myeloid cells, illustrated as slingshot or insect net in the cartoon, can help empower T cells and enable cancer immunotherapy.
(Art by Dr. Sheryl Lu)
Signaling and Therapeutic Targets in Bone Metastasis
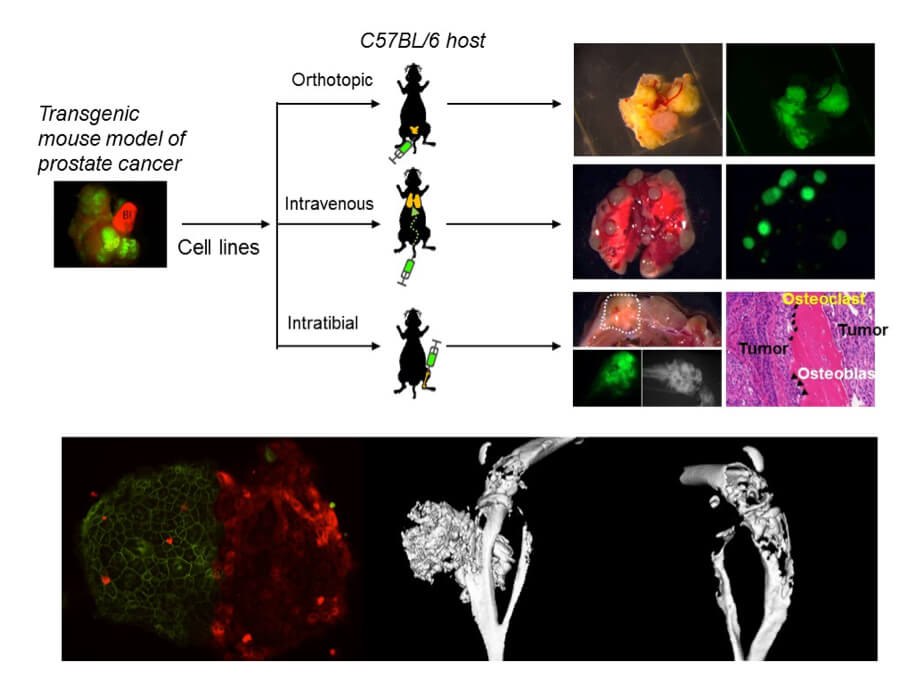
We also have tremendous interests in understanding the signaling and cellular processes pivotal for the formation of osteoblastic bone metastasis by prostate cancer. Prostate cancer is the most commonly diagnosed cancer for men in the United States and most other developed countries, and also showing trend of increased incidence in many developing countries. As the third leading cause of cancer mortality for men in the US, prostate cancer kills patients through relentless metastasis to vital distant organs, with bone being the most frequent targeted site. Unlike bone metastasis in most of other cancer types (e.g. breast cancer, multiple myeloma), prostate cancer generates bone lesions with disorganized osteoblastic features, suggesting the existence of unique signaling mechanisms underlying the aberrant activation of osteoblasts by prostate cancer cells. We are interested in identifying functional drivers and therapeutic vulnerability of prostate cancer bone metastasis through genomic analysis, functional screen and mechanistic investigation. We are currently studying several new prostate cancer genes with the hope that they may emerge as new biomarkers and/or therapeutic targets to treat this deadly disease.
Rare Cancer – New Horizon in Cancer Mechanism and Treatment
Last but not least, we want to emphasize that we are not only interested in a few prevalent cancer types (e.g. prostate cancer, breast cancer), but also very much so in rare cancers. Based on NCI’s definition of rare cancer (incidence rate < 15/100,000), the total cases of rare cancer cases account for 25% of all adult cancers in the USA! Patients with rare cancers also have overall worse survival than those with common cancers. Compared with common cancers, rare cancers are more frequently diagnosed for young individuals. Despite overwhelming clinical significance, rare cancers as a group are much less studied, resulting in often very limited therapy options for patients with rare cancer. As part of the Center for Rare and Neglected Diseases (CRND), our mission is to mechanistically understand and effectively treat focused rare cancer types through bench-to-bedside translational research and partnership with drug discovery powerhouses. To achieve this goal, we use integrated approaches centered at cancer genome mining and functional validation using sophisticated inducible transgenic mouse modeling. For example, one of our focused rare cancer types is penile cancer (squamous cell carcinoma of the penis), which is uncommon in the USA and Europe yet accounts for over 10% of malignancies affecting men in some other countries such as Puerto Rico, Brazil, Uganda and Vietnam. We have established the first set of genetically engineered mouse models of penile cancer which helped reveal mechanisms of chemoresistance of the disease. Ongoing research will further illuminate new therapeutic modalities and combination therapy strategies to treat penile cancer. We are also partnering with a few other groups in the department to co-investigate mechanisms and treatments for Von Hippel-Lindau syndrome (VHL), a rare hereditary disease associated with tumors arising in multiple organs including the central nervous system.